|
Natural Product-Inspired Macrocycles for FK506-binding Proteins
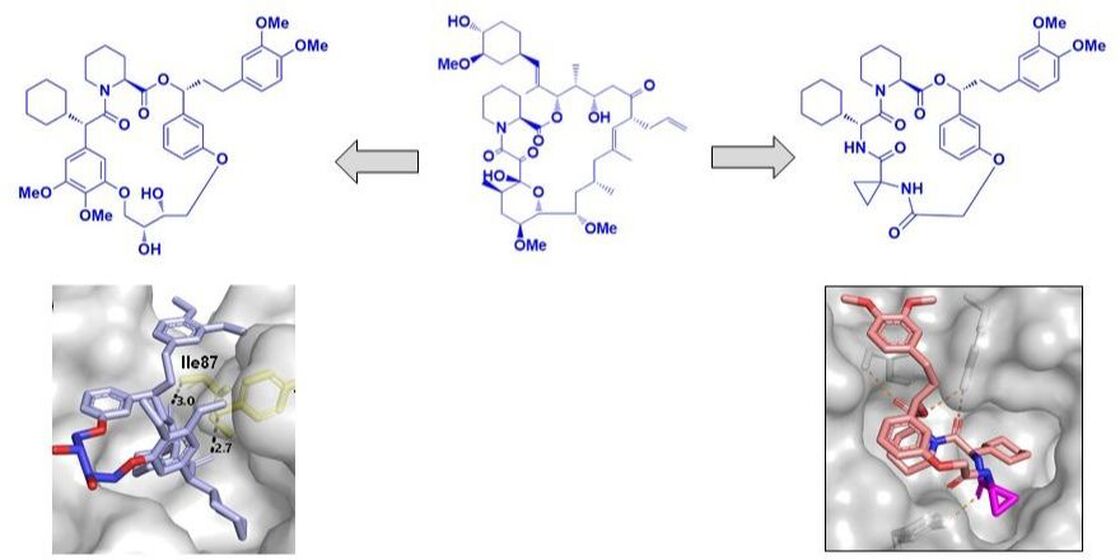
Abstract: Intracellular proteins that have only shallow binding sites represent a substantial challenge for the development of drug-like small molecules. Nature has repeatedly evolved macrocycles to address such difficult-to-drug targets and macrocycles have been suggested as preferred modalities to maintain drug-like properties in the beyond rule-of-five space (bRo5). Here, we present the development of two classed of macrocyclic ligands for the FK506-binding protein 51 (FKBP51), which plays a key role in the human stress response is a potential target for depression, obesity and chronic pain. Starting from the natural products FK506 and rapamycin, we identified the core scaffold as well as a motif conferring strong selectivity for the homolog and counter-target FKBP52. Inspired by the natural products and supported by available co-crystal structures, we engage on two macrocyclization campaign to optimize the initial tool compounds. Side chain functionalization of the linkers employed turned out to be crucial to improve properties. One series revealed a new transient binding mode, which imparted unprecedented selectivity for the homologs FKBP12 and 12.6. In a second series, polar groups in the linker were crucial for potent intracellular target engagement. Taken together, our results emphasize role of linkers and their side chain functionalities in macrocycles. |
Related publications:
Selective Inhibitors of the FK506-Binding Protein 51 by Induced Fit, S. Gaali, A. Kirschner, S. Cuboni, J. Hartmann, C. Kozany, G. Balsevich, C. Namendorf, P. Fernandez-Vizarra, C. Sippel, A.S. Zannas, R. Draenert, E. B. Binder, O. F. X. Almeida, G. Rühter, M. Uhr, M. V. Schmidt, C. Touma, A. Bracher, F. Hausch, Nat. Chem. Biol., 2015, 11, 33-37.
Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors, M. Bauder, C.Meyners, P. L. Purder, S. Merz, W. O. Sugiarto, A. M. Voll, T. Heymann, F. Hausch, J. Med. Chem. 2021, 64, 3320 –3349.
Macrocyclic FKBP51 Ligands Define a Transient Binding Mode with Enhanced Selectivity, A. Voll, C. Meyners, M. Taubert, T. Bajaj, T. Heymann, S. Merz, A. Charalampidou, J. Kolos, P. Purder, T. Geiger, P. Wessig, N. Gassen, A. Bracher and F. Hausch, Angew. Chem. Int. Ed. 2021, 60, 13257–13263.
Development of NanoBRET-Binding Assays for FKBP-Ligand Profiling in Living Cells, M. T. Gnatzy, T. M. Geiger, A. Kühn, N. Gutfreund, M. Walz, J. M. Kolos, F. Hausch, Chem.Bio.Chem, 2021, 22, 2257-2261.
Selective Inhibitors of the FK506-Binding Protein 51 by Induced Fit, S. Gaali, A. Kirschner, S. Cuboni, J. Hartmann, C. Kozany, G. Balsevich, C. Namendorf, P. Fernandez-Vizarra, C. Sippel, A.S. Zannas, R. Draenert, E. B. Binder, O. F. X. Almeida, G. Rühter, M. Uhr, M. V. Schmidt, C. Touma, A. Bracher, F. Hausch, Nat. Chem. Biol., 2015, 11, 33-37.
Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors, M. Bauder, C.Meyners, P. L. Purder, S. Merz, W. O. Sugiarto, A. M. Voll, T. Heymann, F. Hausch, J. Med. Chem. 2021, 64, 3320 –3349.
Macrocyclic FKBP51 Ligands Define a Transient Binding Mode with Enhanced Selectivity, A. Voll, C. Meyners, M. Taubert, T. Bajaj, T. Heymann, S. Merz, A. Charalampidou, J. Kolos, P. Purder, T. Geiger, P. Wessig, N. Gassen, A. Bracher and F. Hausch, Angew. Chem. Int. Ed. 2021, 60, 13257–13263.
Development of NanoBRET-Binding Assays for FKBP-Ligand Profiling in Living Cells, M. T. Gnatzy, T. M. Geiger, A. Kühn, N. Gutfreund, M. Walz, J. M. Kolos, F. Hausch, Chem.Bio.Chem, 2021, 22, 2257-2261.